Clarkson University Research Highlights New Technology that Advances Rapid and Reliable Development of New Diagnostic Tests
Clarkson University’s Milton Kerker Chair of Chemistry Evgeny Katz and Research Assistant Professor Oleh Smutok have recently published a paper in the prestigious scientific journal Nature Nanotechnology reporting their research results that fosters rapid and reliable development of new diagnostic tests.
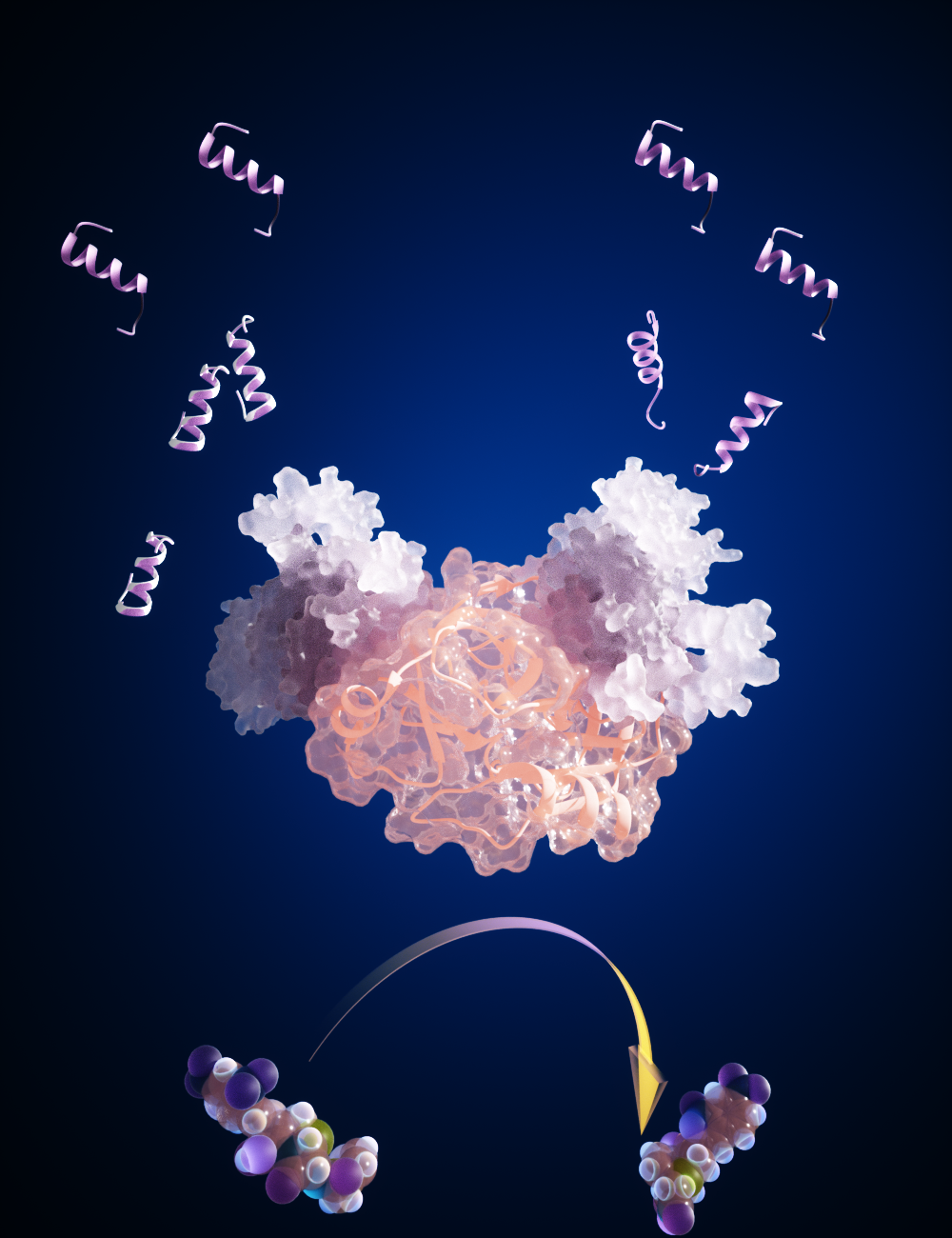
Working in collaboration with Kirill Alexandrov, a professor at Queensland University of Technology in Australia, Katz and Smutok have developed a new approach for designing molecular on/off switches based on artificial allosteric proteins which can be used in a multitude of biotechnological, biomedical and bioengineering applications.
Clarkson’s team, Professor Evgeny Katz and Research Assistant Professor Oleh Smutok (in collaboration with Professor Kirill Alexandrov, Queensland University of Technology, Australia), has developed a new approach for designing molecular ON-OFF switches based on artificial allosteric proteins which can be used in a multitude of biotechnological, biomedical and bioengineering applications. The most important scientific results have been published recently in Nature Nanotechnology (https://doi.org/10.1038/s41565-023-01450-y) - one of the most prestigious journals (impact factor 40.52). The paper titled “Development of epistatic YES and AND protein logic gates and their assembly into signaling cascades” demonstrated that protein switches could be engineered in a predictable way. The research team demonstrated that this novel approach allows them to design and build ultrasensitive and accurate diagnostic tests and biosensors for detecting diseases, analysis of medication efficacy, monitoring the water quality, and detecting environmental pollutants.
The presently available ‘point of care’ diagnostic tests and biosensors, which provided immediate results, such as blood glucose, pregnancy, and COVID test kits, used protein-sensing systems to detect the presence of sugar, pregnancy hormones, and COVID proteins. However, the used technology represents only a tiny fraction of what is needed in a patient-focused healthcare model. Developing new sensing systems is a challenging and time consuming trial-and-error process. The new ‘protein nano-switch’ method developed by the team and published in the present Nature Nanotechnology paper can massively accelerate development of similar diagnostics by decreasing the time and increasing the success rate. It uses proteins modified to behave like ON/OFF switches in response to specific targets. The advantage of the developed approach is that the system is modular, similar to building with Lego bricks, so the parts can be replaced easily to target something else – another drug or a medical biomarker, for example. The method offered the possibility of building many different diagnostic and analytic tests, with a wide range of possible applications including diagnostics of human and animal physiological states, analytical kits for water contamination, and detecting rare earth metals in environmental samples to direct mining efforts. To demonstrate the technology efficacy, the team focused on a cancer chemotherapy drug that is toxic and requires constant measurement to ensure patient welfare. Too little of the drug won’t kill the cancer, but too much could kill the patient. The optical sensor the team designed for the drug uses a color change of chromogenic substrate to identify and quantify the target analyte. Simultaneously, electrochemical approaches, using the same allosterically regulated proteins, have been developed and their advantages compared to common analytical methods have been demonstrated. Both developed sensors were tested on human biofluids demonstrating their efficacy in constant drug monitoring for optimization of the drug concentration during patients' treatment. Thus, the protein-engineering technology developed by the research team provided a novel means to create laboratory tests. This has the potential to improve and expand laboratory testing, which will result in substantial health and economic benefits. The team emphasized: “It’s really exciting, because it’s the first time an artificially designed protein biosensor may be actually suitable for a real-life diagnostic application.” The next step of the planned research will be the use of the developed technology in clinical settings. Then, the team will demonstrate the scale and potential of the technology by building many switches for different diagnostic applications. The team is currently modifying existing proteins, but in the future they can use the same principles to develop components that do not exist and will be designed from scratch. The new technique provides scientists with unprecedented control over the construction of protein-based sensing systems.
These advancements were made possible by an international and interdisciplinary team and excellent teamwork. Notably, Clarkson’s team, Prof. Katz and Dr. Smutok, was mostly working on experimental realization of the biosensing, particularly using electrochemical and optical methods, while the Australian team led by Prof. Alexandrov was mostly working with synthetic biology methods. The research has been supported by the international funding agency Human Frontier Science Program (HFSP) with a multi-million award shared with the Australian group. The research results appeared to be interesting for the US Department of Defence (DoD), which supported the Clarkson-Australia team with a scientific grant for developing specific biosensors. Presently, Clarkson's team is additionally supported by an award from the National Science Foundation (NSF). The successful research resulted in 12 papers published by the international team in the most prestigious journals, including other papers in Nature Communications (2021), Angewandte Chemie (2022), etc. The graduate students, having received training within the framework of this project, obtained postdoctoral positions in the most prestigious universities e.g., Stanford Medical School. Overall, the researchers and students working on the project acknowledge the excellent scientific environment provided by Clarkson University.
Dr. Oleh Smutok received his Ph.D. in Biology in 2007 and D.Sc. degree (Habilitation) in 2019 from the Institute of Cell Biology, National Academy of Sciences (NAS) of Ukraine. He was a Senior Researcher at the Institute of Cell Biology, NAS of Ukraine, Lviv, Ukraine (2007-2020) and Associate Professor at the Drohobych Ivan Franko State Pedagogical University, Drohobych, Ukraine (2017-2020). Since 2020, he is Research Assistant Professor in the Department of Chemistry & Biomolecular Science, Clarkson University, Potsdam, New York, USA. He has (co)authored over 90 papers in high-level journals like: Nature Communications, Angewandte Chemie, Biosensor & Bioelectronics, etc.
Prof. Evgeny Katz received Ph.D. in Chemistry from Frumkin Institute of Electrochemistry, Russian Academy of Sciences, in 1983. He was a senior researcher in the Institute of Photosynthesis in 1983-1991. In 1992-1993 he performed research at München Technische Universität as a Humboldt fellow. Later, in 1993-2006, Dr. Katz was a Research Associate Professor at the Hebrew University of Jerusalem. Since 2006 he is the Milton Kerker Chaired Professor at the Department of Chemistry and Biomolecular Science, Clarkson University, USA. He has (co)authored over 520 papers, Hirsch-index 94. His scientific interests are in the broad areas of bioelectronics, biosensors, biofuel cells, and biocomputing.
